After an exciting Tour de France last month, let’s take a step back to explore another important contest – the race for the superior battery. Lithium-ion (Li-ion) batteries have emerged as the champions of portable electronics and electric vehicles, delivering impressive energy density and long-lasting power. The electrolyte, a critical component of these batteries, plays a vital role in enabling the flow of ions between electrodes. In recent years, researchers have been pushing the boundaries, exploring different electrolyte types, including liquid, solid-state, and quasi- or semi-solid state, each with its own strengths and challenges, much like the Tour de France teams.
Source: Wikimedia commons
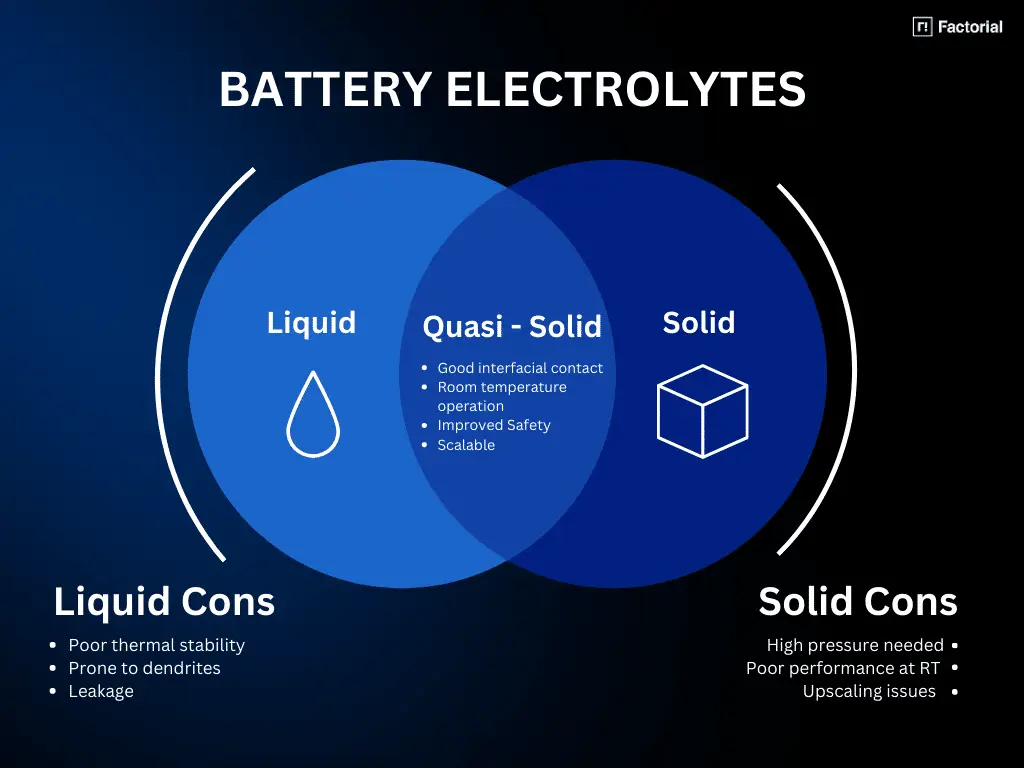
In the quest to develop safer and more efficient batteries, the choice of electrolyte plays a crucial role. Conventional liquid electrolytes offer high ionic conductivity, ensuring efficient charge and discharge processes, but they pose safety risks due to their high flammability. Moreover, conventional liquid electrolytes cannot be used with lithium metal anodes, which are needed to achieve the highest energy densities. On the other hand, solid-state electrolytes provide improved safety and dendrite suppression, but they face challenges in achieving high room temperature conductivity and scalable manufacturing processes. Quasi-solid electrolytes offer a promising middle ground, combining safety benefits with good room temperature performance.
Let’s delve into the characteristics and considerations of each electrolyte type:
Liquid electrolytes – the current champion, but prone to accidents
Liquid electrolytes are the most common type of electrolytes used in commercial li-ion batteries for EVs and consumer electronics. They typically consist of organic solvents, such as ethylene carbonate and dimethyl carbonate, with dissolved lithium salts. A separator is needed to allow ions to flow, but not electrical current.
Think of the electrolyte as the highway that positively charged lithium ions travel on to shuttle back and forth between the anode and cathode. Organic solvent based liquid electrolytes make an extremely smooth highway for ions – that is, what chemists would call excellent ionic conductivity. This conductivity allows for efficient charge and discharge processes, leading to optimal battery performance.
Organic solvents have carbon-based molecules with a partial positive and partial negative charge distribution due to differences in electronegativity between carbon and other atoms. This polarity allows them to interact with ions, such as lithium ions, present in the electrolyte to form a solvation shell that allows for easy movement of the ions. A good analogy is the snug Lycra outfits cyclists wear. The solvent molecules act like the fabric of the Lycra outfit, forming a close-fitting and dynamically adjustable layer around the ions helping the lithium ions to dissolve and stay in solution. Additionally, the solvation shell can affect the solute’s mobility, allowing it to move freely within the solvent.
However, a significant drawback of liquid electrolytes is their high flammability. Not unlike gasoline or alcohol, the organic solvents used are highly combustible, posing safety risks, especially in cases of thermal runaway or battery puncture.
The majority of liquid electrolytes are unsuitable for lithium metal anodes as they do not effectively inhibit the formation of dendrites and do not possess the correct chemical makeup to work with the metal, which can lead to short circuits and battery failure.
All Solid-State Electrolytes: promising, but not ready for the yellow jersey
All solid-state electrolytes have gained attention for their potential to address safety concerns associated with liquid electrolytes. Made of solid materials, typically ceramics or polymers, they eliminate the flammable liquid component, enhancing battery safety.
Moreover, all solid-state electrolytes offer improved resistance to dendrite formation. This property is crucial when using lithium metal anodes, as dendrites can grow and penetrate the separator, causing short circuits. All Solid-state electrolytes to some extent act as a physical barrier, preventing dendrite growth and enhancing battery lifespan.
While all solid-state electrolytes offer exciting potential, there are some challenges that need to be addressed:
- Interface contact: The ions need to move in and out of the electrodes. While liquids naturally conform to the solid material of the electrodes, solid electrolytes may require careful engineering to prevent gaps that can develop after assembly.
- Ionic conductivity at room temperature: Solutions to interface challenges, such as polymer-based solid electrolytes (think of a piece of flexible plastic) have been developed. Though promising, these can have limited ionic conductivity at room temperature, necessitating further optimization to function efficiently without elevated temperatures.
- Manufacturing at scale: Solid electrolytes introduce new requirements to the battery manufacturing process. While different from liquid electrolytes and potentially more complex, ongoing research and development efforts are focused on streamlining these processes to make large-scale production more feasible.
Quasi-Solid-State Electrolytes: The up-and-coming breakout star
Semi-solid or quasi-solid electrolytes, also known as gel or hybrid electrolytes, aim to combine the safety advantages of solid-state electrolytes with the improved performance and manufacturability of liquid electrolytes. These electrolytes are composed of a solid matrix infused with a liquid or gel-like electrolyte. The solid matrix provides structural and thermal stability (thus usable with lithium metal anodes) while the liquid or gel component facilitates ion transportation, flexibility and interface ‘snugness.’
Quasi- or semi-solid electrolytes offer a good compromise, providing enhanced safety compared to conventional liquid electrolytes. They exhibit reduced flammability due to the immobilization of the liquid component within the solid matrix. Importantly, they can provide high ionic conductivity at room temperature compared to traditional solid electrolytes.
In addition, batteries with quasi-solid-state electrolytes should be easier to manufacture than solid-state batteries in the short term because they share major materials, manufacturing equipment, and processes with liquid-based batteries that are already commercialized.
In the race to electrify vehicles, all batteries are critical to the mission of decarbonizing transportation. We are at the forefront of one of the many new advancements in battery technology that are paving the way for a new era: where the world is focused on generating clean, renewable energy.
At Factorial, we are committed to creating the best solution for batteries using the latest technology advancements. Our proprietary FEST® (Factorial Electrolyte System Technology) leverages a quasi-solid-state material and incorporates a lithium metal anode, enabling a significant increase in energy density. The technology has been scaled in 100Ah+ cells, works at room temperature, and is largely compatible with existing lithium-ion battery manufacturing equipment. We are actively working on improving the technology and continuing to scale upwards on the road to commercialization.